Mate recognition is crucial to exchange genetic variations to maintain phenotypic plasticity during evolution. Precise recognition of suitable mates is mediated through sensory perceptions to recognize conspecific and reproductive mates. With computation of sensory stimuli, animals then make decision on behavioral outputs to select the suitable mates. In our current study, we have shown that C. elegans males are able to recognize suitable mates through physical contacts, and change the behavioral states to increase the chance to mate with hermaphrodites. Apart from the decision-making, we notice that the alteration of behavioral states is sustained without further stimuli, which represent the persistent state, which is the fundamental element of high cognitive functions such as emotions. We then study the neural mechanisms of decision-making and persistency of behavioral state. Starting with the simple nervous system in C. elegans, which only have 302 neurons in hermaphrodites, we anticipate these studies of decision-making and persistency of behavioral changes in mate recognition can help us to better understand the brain functions in complex organisms.?
What is the neural mechanism behind the decision-making?
Although the decision-making of animals’ behaviors has drawn a lot of attentions in neuroscience, the genes and cellular mechanisms controlling decision-making have not been satisfyingly understood. The main difficulty is that the mechanistic details are not sufficiently revealed at single cell level due to the limit of tools in animal models. We found that C. elegans males are able to recognize suitable mates through physical contact, and we want to study the underlying neural mechanisms. By using a novel cGAL-UAS system, the Chen lab disseminate the neural mechanisms of decision-making of mate recognition in C. elegans.
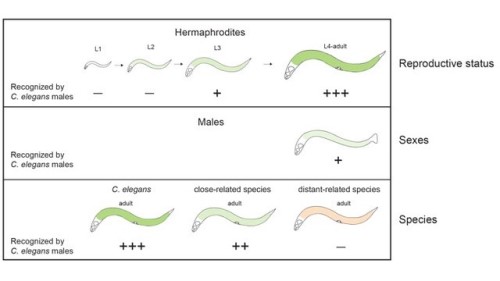
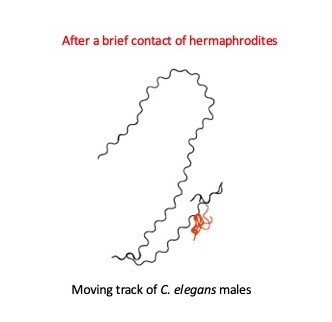
How is the behavioral state sustained by modulation of neural state?
High cognitive functions such as emotions require persistency of behavioral states in high order organisms, where sensory perceptions or behavioral outputs are altered for a certain period to meet the environmental stimuli and internal state. For example, the chance you get the candies from your parents is different, which is likely higher when they are happy. It is a reversible change of neural states as the loss of potential stimuli is accompanied with the fade of the neural states. This persistent state efficiently shorten the responsive time for future stimuli; however, the genetic and neural mechanisms are not fully understood. In our preliminary data, we found that males drastically change its locomotion patterns after a brief contact of hermaphrodites, and this change will sustain for at least 5 minutes. We will use advanced genetics and technology to tackle this question for the persistency of neural state in C. elegans.
Publications
1. Weng JW and Chen CH. (2024) Adult-specific collagen COL-19 is dispensable for contact-mediated mate recognition in Caenorhabditis elegans. MicroPublication Biology. (10.17912/micropub.biology.001141)
2. Nava S, Palma W, Wan X, Oh JY, Gharib S, Wang H, Revanna J, Tan M, Zhang M, Liu J, Chen CH, Lee J, Perry B, Sternberg P. (2023) A cGAL-UAS bipartite expression toolkit for Caenorhabditis elegans sensory neurons. PNAS. 120 (51) e2221680120.
3. Weng, J., Park, H., Valotteau, C., Chen, R., Essmann, C. L., Pujol, N., Sternberg, P. W., & Chen, C. (2023). Body stiffness is a mechanical property that facilitates contact-mediated mate recognition in Caenorhabditis elegans. (Link)
4. Chen CH#, Pan CL#. Live-Cell Imaging of PVD Dendritic Growth Cone in Post-Embryonic C. elegans. (2021) STAR Protocols. 2: 100402 #, corresponding author. (Link)
5. Chen CH, Hsu HW, Chang YH, Pan CL. (2019) Adhesive L1CAM-Robo Signaling Aligns Growth Cone F-actin Dynamics to Promote Axon-dendrite Fasciculation in C. elegans. Developmental Cell. 48(2):215-228 (Recommended by FB1000) (Link)
6. He CW, Liao CP, Chen CK, Teuliere J, Chen CH, and Pan CL. (2018) The Polarity Protein VANG-1 Antagonizes Wnt Signaling by Facilitating Frizzled Endocytosis. Development. 145(24) (Link
7. Chen CH, He CW, Liao CP, Pan CL. (2017) A Wnt-Planar Polarity Pathway Instructs Neurite Branching by Restricting F-Actin Assembly through Endosomal Signaling. PLOS Genet. 13: e1006720 (Recommended by FB1000) (Link)
8. Chen YC, Chen HJ, Tseng WC, Hsu JM, Huang TT, Chen CH, Pan CL. (2016). A C. elegans Thermosensory Circuit Regulates Longevity through crh-1/CREB-Dependent flp-6 Neuropeptide Signaling. Developmental Cell. 39: 209-223. (Link)
9. Chen CH, Lee A, Liao CP, Liu YW, Pan CL., (2014) RHGF-1/PDZ-RhoGEF and Retrograde DLK-1 Signaling Drive Neuronal Remodeling on Microtubule Disassembly. Proc Natl Acad Sci U S A. 111:16568-73. (Link)
10. Hsu JM*, Chen CH*, Chen YC, McDonald KL, Gurling M, Lee A, Garriga G, Pan CL. (2014) Genetic Analysis of a Novel Tubulin Mutation that Redirects Synaptic Vesicle Targeting and Causes Neurite Degeneration in C. elegans. PLoS Genet. 10:e1004715. *equal contribution (Link)
11. Chen CH, Chen YC, Jiang HC, Chen CK, Pan CL. (2013) Neuronal Aging: Learning from C. elegans. J Mol Signal. 8:14. (Invited Review) (Link)
12. Pan CL, Peng CY, Chen CH, McIntire S. (2011) Genetic Analysis of Age-dependent Defects of the Caenorhabditis elegans touch receptor neurons. Proc Natl Acad Sci U S A. 108:9274-9. (Link)
13. Tsai KW, Chang SJ, Wu HJ, Shih HY, Chen CH, Lee CY. (2008) Molecular Cloning and Differential Expression Pattern of Two Structural Variants of the Crustacean Hyperglycemic Hormone Family from the Mud Crab Scylla olivacea. Gen Comp Endocrinol.159(1):16-25. (Link)